SaMD or Software as a Medical Device – Technical Guide to Medical Software
- August 7, 2024
Table of Contents
Software as a Medical Device (SaMD) has revolutionized healthcare, enabling innovative solutions for diagnosis, treatment, and patient monitoring. This technical guide delves into the intricacies of medical software, providing essential insights into its development, regulation, and implementation. By understanding the unique challenges and opportunities presented by SaMD, healthcare professionals and developers can collaborate effectively to create safe, effective, and reliable software solutions that improve patient outcomes.
What is a Software as a Medical Device (SaMD)?
Software as a Medical Device (SaMD) refers to software intended to be used for one or more medical purposes without being part of a hardware medical device. This includes software designed to diagnose, prevent, monitor, treat, or manage diseases or conditions. Unlike traditional medical devices, SaMD operates independently on various technology platforms, from general-purpose computers to smartphones and cloud-based services.
The rise of Software as a Medical Device has prompted regulatory bodies worldwide to develop specific guidelines to ensure safety and effectiveness. The International Medical Device Regulators Forum (IMDRF) has been instrumental in this effort, creating a harmonized framework for Software as a Medical Device regulation. This framework includes definitions, risk categorization, quality management systems, and clinical evaluation standards tailored for software operating independently of any physical medical device.
Regulatory agencies, such as the FDA, play a crucial role in overseeing Software as a Medical Device, providing guidance to developers to navigate the complex regulatory landscape. The FDA’s Digital Health Center of Excellence supports innovation in this field while ensuring patient safety through stringent evaluation and monitoring processes.
Software as a Medical Device can encompass a wide range of applications, from mobile apps that perform complex medical calculations to software that analyzes medical images for diagnostic purposes. The flexibility and rapid development cycles of software allow for continuous updates and improvements, which is both an advantage and a regulatory challenge.
As Software as a Medical Device continues to evolve, stakeholders, including developers, healthcare providers, and regulators, must collaborate closely to balance innovation with the imperative of patient safety. Ensuring that Software as a Medical Device products meet rigorous standards is essential for maintaining trust and efficacy in the increasingly digital landscape of healthcare.
Try it - Love it - Buy it
Avail Our Free Trial Now!
What are the Benefits of a Software as a Medical Device (SaMD)?
Software as a Medical Device (SaMD) refers to software intended to be used for medical purposes without being part of a hardware medical device. This category of software is transforming the healthcare industry by offering numerous advantages. Here are eight key benefits of SaMD:

1. Enhanced Patient Engagement:
SaMD enables patients to actively manage their health by providing real-time feedback and monitoring. For instance, patients with chronic conditions like asthma can use SaMD to track their symptoms and medication adherence, leading to better health outcomes and improved quality of life.
2. Improved Diagnostic Accuracy:
SaMD can leverage advanced algorithms and artificial intelligence to enhance diagnostic accuracy. Tools like AI-powered imaging software can outperform human clinicians in detecting diseases such as cancer at an early stage, potentially saving lives through timely intervention.
3. Cost Reduction in Healthcare:
By automating routine tasks and optimizing workflows, SaMD reduces the cost of healthcare delivery. This efficiency allows healthcare providers to focus more on patient care, ultimately leading to significant cost savings for both providers and patients.
4. Regulatory Compliance and Safety:
SaMD is subject to stringent regulatory oversight by bodies such as the FDA. This ensures that these software products meet high standards of safety and reliability, protecting patient health and increasing trust in digital health solutions.
5. Continuous Improvement and Innovation:
SaMD can be updated and improved more rapidly than traditional medical devices. This flexibility allows developers to quickly implement user feedback, incorporate new scientific discoveries, and enhance software functionality, driving continuous innovation in healthcare.
6. Accessibility and Convenience:
SaMD can be deployed on widely accessible platforms such as smartphones and tablets, making advanced medical diagnostics and monitoring tools available to a broader population, including those in remote or underserved areas.
7- Enhanced Data Collection and Analysis:
SaMD products can continuously collect and analyze patient data, providing valuable insights into health trends and treatment efficacy. This data-driven approach supports personalized medicine and can improve overall healthcare delivery.
8- Support for Clinical Decision-Making:
SaMD provides healthcare professionals with sophisticated decision-support tools that can analyze large datasets, recognize patterns, and suggest optimal treatment plans. This support enhances clinical decision-making, leading to better patient outcomes.
In conclusion, Software as a Medical Device offers numerous benefits that enhance patient care, improve diagnostic accuracy, reduce healthcare costs, and drive continuous innovation in the medical field. Its ability to be rapidly updated and deployed on accessible platforms makes it a crucial tool in modern healthcare.
How Can Businesses Benefit From SaMD?
Software as a Medical Device (SaMD) is transforming the healthcare industry, providing numerous benefits to businesses. By integrating Software as Medical Device, companies can enhance patient outcomes and streamline operations. For instance, Software as Medical Device can perform complex medical functions such as diagnosing conditions, suggesting treatments, and managing clinical data. This not only improves accuracy but also reduces the workload on healthcare professionals, allowing them to focus on patient care.
Furthermore, Software as Medical Device offers significant cost savings for businesses. By automating various diagnostic and treatment processes, companies can minimize the need for physical devices and reduce operational costs. Software as Medical Device applications can be easily updated and maintained, ensuring that businesses stay compliant with the latest regulations and medical standards. This flexibility allows for continuous improvement and adaptation to new medical findings, keeping the business at the forefront of medical technology.
Another crucial advantage of Software as Medical Device for businesses is the ability to gather and analyze large amounts of health data. This data can be used to gain insights into patient health trends, improve treatment protocols, and develop new products and services. Businesses can leverage this data to create personalized treatment plans, enhance patient satisfaction, and build a competitive edge in the market. Additionally, real-time data analysis helps in monitoring the performance of medical devices, ensuring their optimal functionality and safety.
Software as Medical Device also facilitates better regulatory compliance and faster market entry. The FDA and other regulatory bodies are developing frameworks to streamline the approval process for Software as Medical Device. Businesses that adopt Software as Medical Device can benefit from these expedited processes, allowing them to bring innovative products to market more quickly. This agility is critical in the fast-evolving healthcare landscape, enabling companies to respond promptly to emerging health challenges and opportunities.
Moreover, Software as Medical Device enhances interoperability within the healthcare ecosystem. It can integrate seamlessly with other medical devices and health IT systems, enabling comprehensive patient care and efficient data sharing. This interoperability is vital for creating a connected healthcare environment where patient data flows smoothly across different platforms, enhancing coordination and reducing errors.
In conclusion, Software as a Medical Device (SaMD) offers businesses numerous benefits, including improved patient outcomes, cost savings, enhanced data analytics, better regulatory compliance, and increased interoperability. By embracing Software as Medical Device, companies can drive innovation, improve efficiency, and maintain a competitive edge in the healthcare industry.
Data Security Regulations For Software as a Medical Device (SaMD):
Software as a Medical Device (SaMD) has emerged as a pivotal technology in modern healthcare, offering innovative solutions for diagnosis, treatment, and patient management. However, the integration of software into medical applications introduces significant data security concerns. To ensure the safety and privacy of patient data, several data security regulations have been established. Here are nine key regulations critical to the development and deployment of Software as Medical Device.
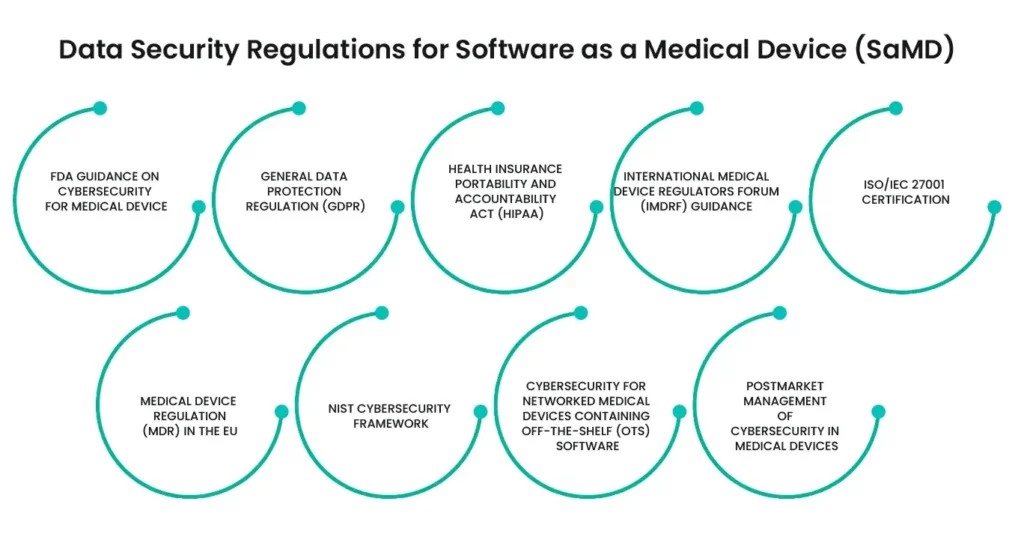
1- FDA Guidance on Cybersecurity for Medical Devices:
The FDA provides guidelines on managing cybersecurity in medical devices, emphasizing the importance of safeguarding patient data from cyber threats. These guidelines cover risk management, vulnerability identification, and mitigation strategies throughout the software lifecycle.
2- General Data Protection Regulation (GDPR):
GDPR imposes strict data protection requirements for software that processes personal data of EU citizens. Software as Medical Device developers must ensure compliance by implementing robust data protection measures, obtaining explicit consent, and ensuring data portability and erasure rights.
3- Health Insurance Portability and Accountability Act (HIPAA):
In the United States, HIPAA sets national standards for the protection of sensitive patient health information. Software as Medical Device must comply with HIPAA’s requirements for data privacy, security measures, and breach notification protocols to protect electronic health records.
4- International Medical Device Regulators Forum (IMDRF) Guidance:
IMDRF provides a global framework for the regulation of Software as Medical Device, including data security aspects. Their guidelines help ensure that Software as Medical Device products meet consistent safety and performance standards across different regulatory jurisdictions.
5- ISO/IEC 27001 Certification:
Achieving ISO/IEC 27001 certification demonstrates that a Software as Medical Device company has established a robust information security management system (ISMS). This standard helps in systematically managing sensitive data and mitigating risks related to information security.
6- Medical Device Regulation (MDR) in the EU:
MDR regulates the safety and performance of medical devices, including Software as Medical Device. It mandates rigorous clinical evaluations and post-market surveillance, ensuring that Software as Medical Device products maintain high standards of data security throughout their lifecycle.
7- NIST Cybersecurity Framework:
The National Institute of Standards and Technology (NIST) Cybersecurity Framework provides a policy framework of computer security guidance for Software as Medical Device developers. It focuses on identifying, protecting, detecting, responding to, and recovering from cyber threats.
8- Cybersecurity for Networked Medical Devices Containing Off-the-Shelf (OTS) Software:
FDA guidelines for networked medical devices emphasize the importance of securing software that integrates off-the-shelf components. Software as Medical Device must implement stringent security controls to prevent unauthorized access and data breaches.
9- Postmarket Management of Cybersecurity in Medical Devices:
The FDA’s postmarket guidance outlines the responsibilities of Software as Medical Device manufacturers in managing cybersecurity risks after the product has been marketed. It includes monitoring, identifying, and addressing vulnerabilities through updates and patches.
Ensuring data security in Software as Medical Device is paramount to protect patient safety and privacy. Compliance with these nine key regulations helps Software as Medical Device developers create secure, reliable, and effective medical software solutions, thereby fostering trust and advancing healthcare innovation.
Decision-Making Aids for the Qualification or Classification of Software as Medical Device (SaMD):
Decision-making aids for the qualification and classification of Software as Medical Device (SaMD) play a crucial role in the healthcare industry. As defined by the International Medical Device Regulators Forum (IMDRF) and the FDA, Software as Medical Device is intended for medical purposes without being part of any hardware device. The classification of Software as Medical Device involves understanding its intended use, which includes diagnosis, treatment, or prevention of diseases, and ensuring that it meets the criteria set by regulatory bodies.
The decision-making process for determining whether a piece of software qualifies as a medical device involves assessing its medical functionality independently of hardware. This distinction is essential because software that powers or controls a hardware device is categorized differently, often referred to as Software in a Medical Device (SiMD). Understanding these nuances is critical for developers to ensure compliance with regulatory standards and to navigate the approval processes in various global markets, including the US and the EU.
Classification frameworks, such as those provided by the IMDRF, help in categorizing Software as Medical Device based on the significance of information provided and the healthcare situation addressed. These frameworks guide manufacturers in identifying the appropriate risk class for their software, which can range from Class I (low risk) to Class III (high risk) in the US, and similarly tiered classifications in the EU under the MDR. Proper classification impacts the regulatory requirements, including documentation, clinical evaluation, and post-market surveillance.
Regulatory bodies like the FDA and European Commission have established specific guidelines for Software as Medical Device, which include detailed documentation and compliance with standards like IEC 62304 for software lifecycle processes. These guidelines emphasize the importance of maintaining rigorous quality management systems and ensuring cybersecurity to protect patient data and device integrity. Developers must stay updated with these evolving regulations to ensure their software meets the necessary safety and efficacy standards required for medical devices.
In conclusion, decision-making aids for qualifying and classifying Software as Medical Device are essential for ensuring that such software meets regulatory standards and effectively serves its medical purposes. By understanding the definitions, regulatory frameworks, and compliance requirements, developers can successfully navigate the complex landscape of Software as Medical Device and contribute to advancing healthcare technologies.
What are the Framework Regulations for SaMD?
Software as a Medical Device (SaMD) refers to software intended to be used for medical purposes without being part of a hardware medical device. Regulating Software as Medical Device is crucial due to its impact on health outcomes and patient safety. Here are the five key framework regulations for Software as Medical Device:
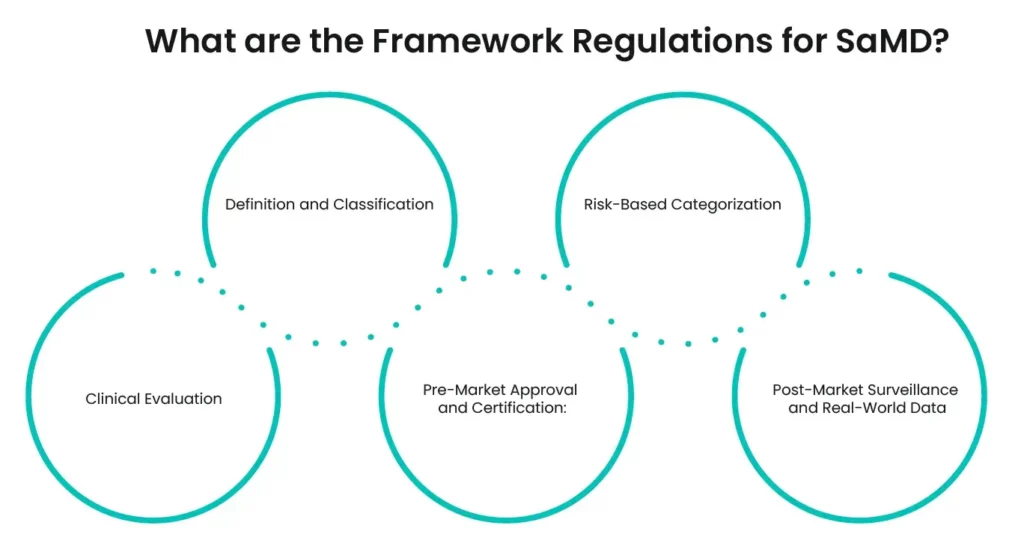
1. Definition and Classification:
The first step in regulating Software as Medical Device is to clearly define and classify what constitutes software as a medical device. The International Medical Device Regulators Forum (IMDRF) provides a comprehensive definition, distinguishing Software as Medical Device from software that merely supports medical devices.
2. Risk-Based Categorization:
Software as Medical Device is categorized based on the level of risk it poses to patients and users. The FDA and other regulatory bodies use a risk-based approach to classify Software as Medical Device into different categories, determining the extent of regulatory control needed. This ensures that higher-risk software undergoes more rigorous scrutiny.
3. Clinical Evaluation:
A thorough clinical evaluation is essential for Software as Medical Device. This involves assessing the software’s clinical functionality and performance to ensure it meets safety and effectiveness standards. The FDA outlines specific guidelines for conducting clinical evaluations of Software as Medical Device.
4. Pre-Market Approval and Certification:
Software as Medical Device developers must obtain pre-market approval or certification from regulatory authorities before launching their products. The FDA’s Pre-Cert program aims to streamline this process for developers who demonstrate a commitment to quality and excellence, facilitating quicker access to market for compliant Software as Medical Device.
5. Post-Market Surveillance and Real-World Data:
The AI personalizes medical history questions based on previous responses, ensuring relevance and improving the quality of the data collected. This adaptability enhances the depth and accuracy of the information gathered.
These framework regulations for software as a medical device (SaMD) ensure that such software is safe, effective, and reliable, safeguarding public health while fostering innovation in the medical software industry.
Examples of Software as a Medical Device (Software as Medical Device):
Software as a Medical Device (SaMD) represents a transformative approach in healthcare, where software is designed to perform medical functions without being part of a hardware device. This paradigm shift leverages advanced technology to diagnose, manage, and treat various medical conditions. Below are ten notable examples of open-source software as a medical device (SaMD), illustrating their application and impact in the medical field.
1- GNU Health:
An open-source health and hospital information management system used for primary care and hospital management. It integrates Electronic Medical Records (EMR), Hospital Information Systems (HIS), and Health Information Systems (HIS).
2- OpenMRS:
A collaborative open-source project that develops software to support the delivery of healthcare in developing countries. It is used for managing patient records and clinical workflows.
3- Epi Info:
Provided by the CDC, this software assists in data collection, epidemiological research, and outbreak investigations. It includes tools for questionnaire design, data entry, and statistical analysis.
4- OsiriX:
An image processing software dedicated to DICOM images, used for viewing and analyzing medical images. It supports 3D and 4D imaging and is widely used in radiology.
5- OpenEMR:
An open-source electronic health records and medical practice management software that provides billing, scheduling, and clinical decision support systems.
6- CaPTk (Cancer Imaging Phenomics Toolkit):
An open-source software platform for the analysis of radiographic images and extraction of quantitative imaging features, supporting cancer research and clinical decision-making.
7- BioImage Suite:
A comprehensive software suite for the analysis of biomedical images, integrating various algorithms for image processing, segmentation, and visualization.
8- ITK-SNAP:
An interactive software for the segmentation of 3D medical images, providing a user-friendly interface for delineating anatomical structures and quantifying tissue volumes.
9- OHIF (Open Health Imaging Foundation) Viewer:
A web-based medical image viewer designed for integrating with PACS (Picture Archiving and Communication System) and other clinical systems, facilitating remote viewing and diagnosis.
10- Cytoscape:
An open-source software platform for visualizing complex networks and integrating these with any type of attribute data, widely used in genomics and systems biology.
The development and implementation of open-source software as a medical device (SaMD) are revolutionizing healthcare by enhancing accessibility, promoting innovation, and improving patient outcomes. These examples demonstrate the potential of Software as Medical Device in various medical applications, from imaging and diagnostics to patient management and research, highlighting the significant contributions of open-source software in the medical domain.
Subscribe to our Newsletter!
Subscribe our Newsletter to stay updated
SaMD Risk Based Categories:
Software as a Medical Device (SaMD) represents a significant evolution in healthcare technology. Unlike traditional medical devices that incorporate software to function, Software as Medical Device refers to software intended for medical purposes that operate independently of any hardware. The classification and regulation of Software as Medical Device are crucial to ensure safety and efficacy, given its growing role in patient care and medical decision-making.
Regulatory Definitions and Frameworks:
Regulatory bodies like the FDA and the International Medical Device Regulators Forum (IMDRF) have defined Software as Medical Device to ensure clear guidelines. According to the FDA, Software as Medical Device is defined as software intended for medical purposes that perform these purposes without being part of a hardware device. The IMDRF provides a similar definition, emphasizing the software’s medical intent and its standalone nature. Understanding these definitions is vital for developers to ensure compliance and proper categorization of their software products.
Risk-Based Categories for SaMD:
The categorization of Software as Medical Device into risk-based categories is essential for determining the level of regulatory scrutiny required. The IMDRF framework categorizes Software as Medical Device based on the significance of the information provided by the software and the state of the healthcare situation or condition. This categorization helps in assessing the potential impact on patient health and safety, guiding regulatory requirements and development processes. The risk levels range from minimal to serious, reflecting the severity of potential outcomes if the Software as Medical Device fails or malfunctions.
Global Regulatory Perspectives:
Different regulatory bodies have their specific guidelines for Software as Medical Device, reflecting the global nature of medical device markets. For instance, the European Union (EU) follows the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which include specific provisions for software. The EU uses terms like Medical Device Software (MDSW) and applies rules like Rule 11 to classify software based on its intended use and potential impact. Understanding these regulations is critical for manufacturers aiming to market their Software as Medical Device products internationally.
Compliance and Safety Standards:
Compliance with international standards, such as IEC 62304, which outlines software lifecycle requirements, is mandatory for SaMD developers. This standard emphasizes a structured approach to software development, maintenance, and risk management, ensuring that SaMD products meet safety and effectiveness criteria. Additionally, adopting agile methodologies within the regulatory framework is feasible and increasingly necessary to keep up with technological advancements and regulatory expectations.
Examples and Practical Applications:
Real-world applications of SaMD illustrate its diverse potential. Examples include software that processes diagnostic images, monitors patient data in real-time, and even mobile apps that assist in managing chronic conditions. These applications demonstrate how SaMD can enhance diagnostic accuracy, improve patient outcomes, and facilitate more personalized healthcare solutions.
In conclusion, the categorization and regulation of Software as Medical Device are pivotal in the modern medical landscape. As SaMD continues to evolve, understanding and adhering to risk-based categories and global regulatory requirements ensure these innovative tools can safely and effectively improve patient care and healthcare delivery.
Read More Blogs
See what’s trending in the medical world with our blogs.




